About Us
Full Health Clarity, Inc. was incorporated in September 2023 with the vision of solving the challenges of deploying the FDA Unique Device Identifier (UDI) not only as a supply chain solution but also to enhance and enrich the lives of patients undergoing surgical procedures, particularly those receiving medical device implants. Those patients with medical device implants will require long-term care and performance tracking and maintenance of their devices. Our vision addresses how to drive rapid adoption of UDI within healthcare delivery organizations of all sizes and types in the short term. Our longer-term vision enables patient medical device longitudinal performance and life impact analytics across all medical device types.

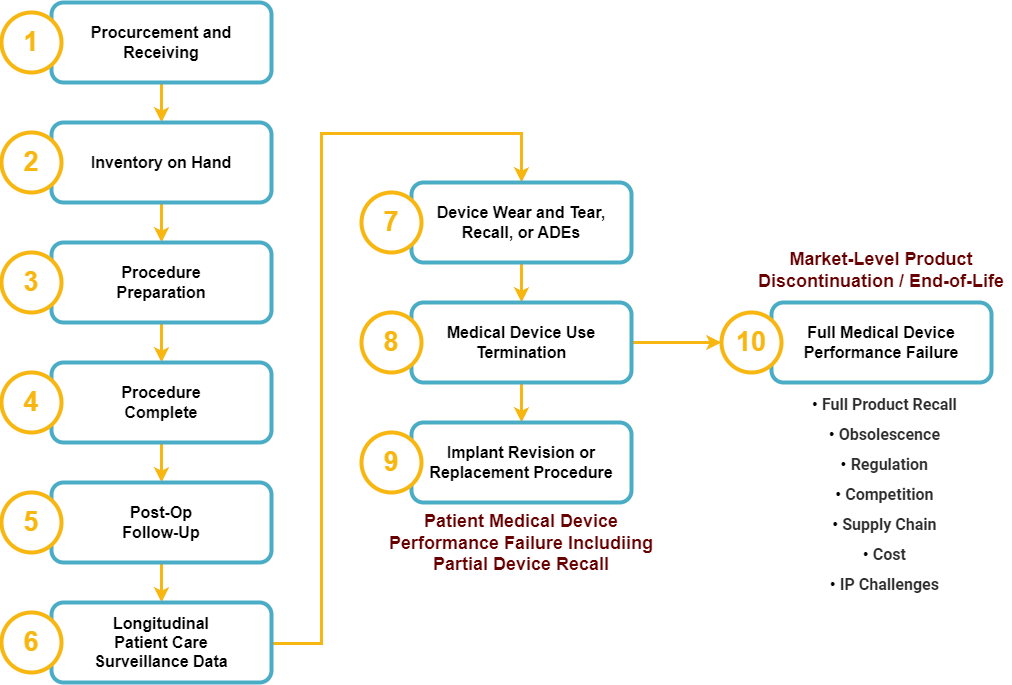
The Complete Medical Device Lifecycle
At Full Health Clarity, Inc., our focus spans the last leg of the medical device journey, from the receipt of newly procured medical device inventory, all the way to the end of the medical device’s useful life. Much of the attention to UDI since it became federal law in 2013 has been on the challenges and issues manufacturers faced when deciding how to serialize their devices. Also, multiple high-profile projects exploring and qualifying the challenges and costs associated with UDI have been undertaken and completed. While these efforts have been critical to the refinement of the UDI regulation and have provided practical experience on how to implement UDI, as noted by leading voice published August 21, 2023, in the JAMA Internal Medicine, “Developing the needed infrastructure is complex and time-consuming; there is currently no ‘out of the box’ solution.”
That’s where Full Health Clarity, Inc. plans to change the game.